Protein Structure
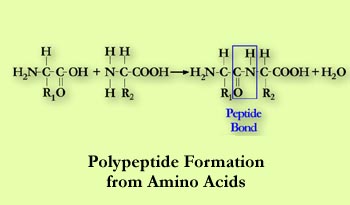
Chemical Structure Proteins contain the elements carbon, nitrogen, hydrogen and oxygen. They are made up of chains of up to twenty-two different amino acids which are joined together by peptide bonds. These are called polypeptides.
Primary Structure of proteins is the sequence of amino acids.Twenty-two amino acids are used in the chain structure of proteins. Amino acids can be linear or ring molecules but all contain an amino (-NH2) and a carboxyl (-COOH) group. Secondary Structure is the folding of the long thin chains of amino acids into structures such as the alpha helix. These structure stabilize the protein since other bonds form as the amino acid chains wrap around each other. Tertiary Structure is the shape of a large portion of the protein molecule which involves additional folding of the helix for example creating even more bonds and stability.
Protein Quality and Sources
At least eight amino acids are considered essential (must be supplied in the diet) for tissue maintenance and growth of adults. These essential amino acids are isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine. The amino acid histidine is essential for the growth and development of children but can be synthesized by adults. Other non-essential amino acids are also required to maintain health but can be synthesized by the body if supplied with a source of nitrogen. These non-essential amino acids are alanine, arginine, aspartic acid, cysteine, cystine, glutamic acid, glycine, hydroxyproline, proline, serine and tyrosine. Biological Values The balance of amino acids in a specific protein determines its biological value. Complete proteins have a high values since they contain adequate amounts of the essential amino acids to promote normal growth in animals. Proteins of high biological value are found in many animal sources such as milk, eggs, cheese, meat, poultry and fish. Plant sources are normally lacking specific essential amino acids and have lower biological scores. Also, compared to animal sources, relatively large amount of plant products (total calories) must be consumed to get enough protein. The exception in plant products is the soybean, which contains relatively large amounts of high biological value protein. True vegetarians must be careful to mix their diet with plant sources from both cereals and legumes to fulfill their daily requirement of essential amino acids. For example, corn protein is seriously deficient in lysine and tryptophan. Legumes and oilseeds are generally deficient in methionine. A balanced protein diet has to be calculated carefully. This is difficult for young children who usually need extra protein from animal sources such as milk. Even a small amount of animal protein greatly enhances the diet quality of mainly vegetarians.
Protein Efficiency Ratios Several tests using rats have been developed to evaluate both the quality and digestibility of proteins. The protein efficiency ratio (PER) is the ratio of weight gain in rats for 1 g of protein consumed. Net Protein Retention is considered a better index, since it takes into account the palatabilty of the protein source. two groups of animals are used - one group on the protein diet and one group on a protein-free diet. The weight loss of the non-protein group is compared to the weight gain of the protein added group. The test properly controlled is independent of food intake.
Health IssuesProtein in excess of body requirements is not stored but is broken down and the nitrogen excreted in the urine. The remainder of the molecule is used by the body for energy, or stored as fat. Protein Deficiency
Protein Excess
Protein Intolerance
|